Aluminium is the second most widely used metal in the world. Engineers prefer aluminium for its light weight, corrosion resistance and reasonable price.
However, untreated aluminium has low wear resistance. On exposure to the environment, it forms a thin aluminium oxide layer naturally that provides aluminium with its characteristic corrosion protection. But this naturally formed oxide film can erode upon reaction with other environmental elements.
The answer to providing better protection lies with anodising. This procedure has other benefits as we will learn further into the article. But let’s start from the beginning.
What Is Anodising?
It is an electrochemical process that develops an aluminium oxide coat on the surface of the part or product. This protects the product from wear and tear while improving the aesthetics. In this process, the product to be coated acts as an anode in an electrolytic cell, hence the name.
On an industrial scale, anodising made its first appearance in 1923.
Soon, many variations of this process came into use for different materials using various electrolytic chemicals. It was around this time that Gowen and O’Brien used sulfuric acid to anodise aluminium.
This process from almost a century ago still remains the most common and effective method today.
Anodised Aluminium Benefits
The aim of the process is to increase the thickness of aluminium oxide on the surface of the product.
Aluminium oxide layer is extremely hard. On the Mohr’s scale, it has a score of 9 and is second in hardness only to diamond. It is so hard that it is commonly used as an abrasive in sandpapers. Depositing a layer of this material on the product ensures that the product will have high wear resistance.
The thickness of this layer depends on the purpose of anodising. For decorative purposes, a thin layer is enough. A thicker layer protects the surface besides improving the appearance.
Having a thick layer of aluminium oxide also makes the metal surface more receptive to dying as pores are created on the surface when it is anodised. Then, desired pigments are introduced that fill the pores from the surface to its very depth. This makes the pigment quite durable as it cannot be scratched away.
Anodising can also act as an excellent primer for a regular coat of paint on the surface instead of accommodating it into the actual oxide layer.
Anodising aluminium improves the insulation properties of aluminium as aluminium oxide is not a good conductor of electricity.
Working Principle
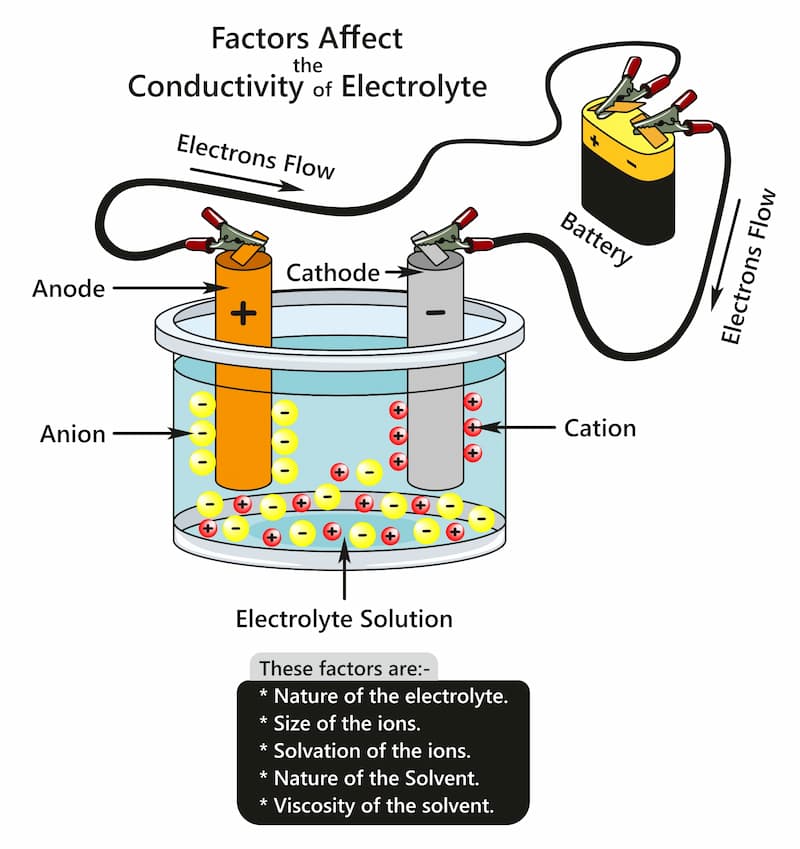
Anodising works on the principle of an electrolytic cell. In this procedure, the anodising tank is filled with a suitable electrolyte. In this tank, the part is usually suspended to expose most of the surface to the electrolyte.
We then place plates of suitable elements (usually lead or aluminium) in the tank. The next step is completing the circuit between the cathode and anode through a power source.
- Personal account manager
- Quality assurance
- Payment terms for companies
- On-time delivery by Fractory
The aluminium product is connected to the positive terminal, and the plates are connected to the negative terminal of the battery. As the circuit is now complete, the current passes through it.
The value and duration of the electrical current passed will determine final features such as the thickness of the aluminium oxide layer on the anodised aluminium product.
Aluminium Anodising Process
Most anodising setups today still use the original sulfuric acid bath for the process. However, many new features have been added to significantly improve the final result in terms of aesthetics and functionality of the product.
The modern aluminium anodising process is very technical. Generally, aluminium anodising consists of the following steps.
ARVE error: url: https://www.googleapis.com/youtube/v3/videos?part=snippet%2Cstatistics&id=0yl35W0o9S0&key=AIzaSyAQ7WFzTAUrOX-FjsIrFS3JwZBFzgIvloc Status code 200 expected but was 403.
Cleaning
The surface of the aluminium product needs cleaning prior to anodising. Exposing the surface uses acidic or alkaline cleaning agents to clean grease/dirt from the surface.
Pre-treatment
This step eliminates any surface imperfections. The goal is to provide a visible finish with a clean and smooth surface. This is done by using two main processes – brightening and etching.
Brightening
Brightening or bright finishing cleans any heavy metal residues left over from the cleaning process. Using a concentrated mixture of nitric and phosphoric acids to chemically smoothen the surface provides a metallic finish ready for anodising.
Etching
Etching removes a layer of aluminium from the product surface to provide a matte finish (see more about gloss levels). A hot solution of sodium hydroxide is used to remove surface imperfections.
Anodising
After pretreatment, the product is ready for anodising. As mentioned above, sulfuric acid is the go-to electrolyte for aluminium anodising. Alternatives that are sometimes used are organic acid, borate, tartrate, phosphoric acid, and chromic acid.
Colouring

There are several methods to add colour to anodised aluminium. Different colours need different methods. Let’s look at two of the most popular methods of colouring anodised aluminium.
Electrocolouring
One of these methods is the electrocolouring method. This method is used for darker shades. In electrocolouring, the anodised aluminium product is introduced to inorganic metallic salts through an electrolyte.
The anodised aluminium product becomes one electrolyte, and graphite (or aluminium) becomes the other. The oxide or hydroxide precipitates in the pores adding colours such as black, brown, blue, yellowish grey, and bronze to the film.
Dyeing
Dyeing is the other popular method of adding colour to an anodised aluminium product. The pores that are formed during the electrochemical process readily absorb dies or pigments.
They fill the pores through the entire thickness of the aluminium oxide layer. Since the thickness of this layer can be up to 50 microns in some cases, this method is quite durable. Scratching or rough usage of the part doesn’t affect the colour due to the layer’s thickness. Also, the range of available colours is wide.
Sealing
Sealing is the final step in the aluminium anodising process. This prevents water leakage and improves corrosion resistance of the anodised aluminium product. There are three methods of doing this – hot method, cold or a combination of the two.
Sealing reduces the chances of staining, scratching, colour degradation and crazing of the surface.
Types of Anodising
Based on the thickness of the aluminium hydroxide layer, there are 2 types of anodising.
Decorative anodising
Decorative anodising, as the name implies, has its focus on providing a nice aesthetic finish first and providing protection as more of a nice extra.
For decorative anodising, the recommended layer is between 5µm to 25µm. To get the best result when dyeing the parts, its best to keep the thickness between 15µm to 25 µm. ISO 7599:2018 specifies the method for decorative anodising for aluminium and its alloys.
Hard anodising
In cases where we need superior protection of aluminium alloys (marine applications or exposure to corrosive chemicals), we recommend opting for hard anodising.
The thickness of the oxidation coating must be between 25µm and 50µm. ISO 10074:2017 provides the specifications for hard anodic oxidation coatings.
Final Finish

Anodising gives the aluminium surface a superior appearance. As we know, the surface consists of the pores with pigments as well as the uncoloured portions where the surface reacts with oxygen to prevent further oxidation. As the light strikes both these surface features at the same time, it interferes on reflection, giving the metal an attractive metallic shine.
The surface also has very few imperfections as it reacts uniformly with the electrolyte giving it a smooth finish.
Anodising vs Powder Coating
Powder coating is a type of surface treatment that is most common for coating steels but also available for aluminium. The surface of aluminium is coated with polyester powder for decoration as well as protection. Manufacturers have a choice between these two methods when they are looking for surface treatment options for aluminium.
Anodising is better than traditional powder coating in many ways, some of which are as follows:
- It is an inorganic finish and provides a superior surface finish compared to organic counterparts such as powder coating.
- When it comes to appearance, anodising has a metallic sheen and is extremely well integrated with the surface compared to powder coating. An anodised surface reacts differently to both natural and artificial light.
- Anodising is also better in the long run. Powder coating sometimes suffers adhesion failure and even if it doesn’t, the colour will fade over time.
What Materials Can Be Anodised?
Besides aluminium, many other metals and even plastics are suitable for anodising.
Metals such as magnesium, titanium, zirconium, niobium, zinc, hafnium, and tantalum are anodised, albeit for different purposes.
In the same way, we can anodise conductive plastics. All anodised products develop superior surface finish, attractive appearance, and generally last longer than their untreated counterparts.
FAQ
What is meant by the barrier layer in anodising?
When aluminium is anodised in an acidic solution, its surface starts to lose aluminium ions. This causes erosion of the aluminium surface and to counter this, the surface reacts with negatively charged oxygen ions in the electrolyte.
While the points where the initial erosion takes place continues to be eroded, the rest of the surface forms an aluminium oxide layer that acts as a barrier against further erosion. This layer is known as the barrier layer. It is quite thin compared to the porous layer formed due to anodising.
Can the entire product surface be anodised?
It is not possible to anodise the entire surface of a product. An electric terminal must be connected to the part throughout the duration of the process, so wherever it is connected, that portion will not be anodised.
In order to minimise the effect of this limitation, the connection is placed at non-critical points. The best place is usually a hidden face on the part.
Hard anodising requires a higher voltage and electrical current. In such cases, the connection is made through a threaded hole in the product for good electrical contact.




