Copper alloys have great material properties useful for many applications. Pure copper, however, has been one of the most important metals for the last 6000 years. Comparing it to other metals, its biggest advantages are:
- Good electric conductivity
- High thermal conductivity
- A remarkable mixture of strength and plasticity
- Corrosion resistance in many environments
Those properties, including diamagnetism, of copper are also present in its alloys. A wide range of alloying elements add further desirable properties. While many copper alloys have suitable characteristics for different applications, they have largely been replaced by aluminium alloys and plastic materials.
The reason for that is copper’s comparatively high price. Of all the different types of metals, copper is pretty high up on the price list. Thus, copper also fetches a high price when scrapped. But it’s important to understand copper scrap grading fundamentals as not all copper is the same, and the price offer depends largely on its grade.
Despite the notable strength and durability of copper alloys, they are not immune to corrosion. The oxidation of copper components in these alloys can lead to colour changes and surface degradation over time, particularly in harsh environments.
Nevertheless, copper alloys, such as brass, bronze and cupronickels, have cemented their places as useful materials in different sectors, including engineering.
Brass

Adding zinc to copper strengthens the alloy because of zinc’s ability to be dissolved. At the same time, the copper alloy’s plasticity increases, which is an unusual feature.
10…20% Zn alloys are known as gilding metals that are used in the jewellery industry and in the production of heat exchangers. 30% Zn alloys are called cartridge brass for a pretty self-explanatory reason. The upper threshold of zinc in formable brass is around 35%.
Adding other alloying elements can further improve brass’s properties. Sn and Al, for example, increase its corrosion resistance in seawater.
Single Phase Brass

Applications: Jewellery, art, deep drawn parts (cutlery, musical instruments, etc) and ammunition cartridges.
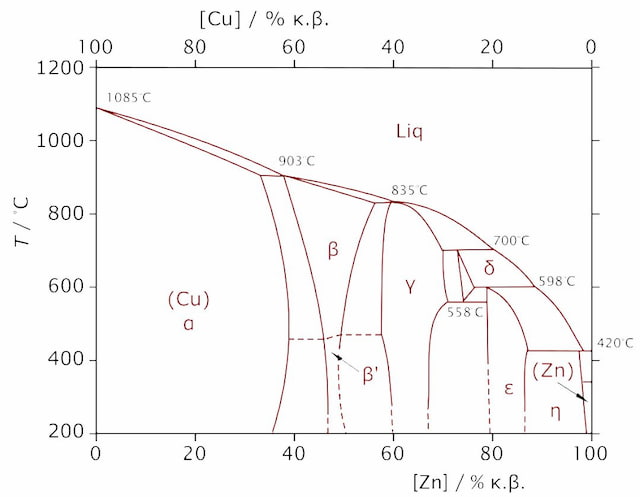
Single phase brasses contain Zn up to 37%. Those are called alpha brasses. Single phase brasses have a homogeneous crystal structure.
Such brasses are softer and have higher ductility. Those qualities make alpha brasses suitable for cold working, drawing, bending, etc.
The aforementioned cartridge brass (70/30 Cu/Zn) falls into this category. Because of the suitability for cold drawing, it’s the ideal candidate for producing ammunition shells in large quantities without high energy requirements.
Its resistance to corrosion, compared to brass with higher zinc content, makes single phase brass suitable for producing different types of fasteners.
Double Phase Brass

Applications: Heat exchangers, capacitors, parts made by automatic cutting benches, etc.
Double phase brasses, also known as duplex brasses, contain both the α and β phases. Thus, both alpha grain structure and beta grain structure are present.
Double phase brass is more affordable than single phase brass because of the larger amount of zinc used in it. At the same time, it’s more prone to corrosion. Still, the chemical composition leads to greater strength and hardness. Therefore, double phase brass is suitable for hot forming and casting. Metal extrusion, stamping and die casting are usable methods with that type of metal.
The most used alpha-beta brasses have a Cu/Zn relationship of 60/40. Such brasses are known as Muntz metals. In order to improve material properties, double phase brasses have more alloying elements. Small amounts of Pb increase the material’s cutting properties. Mn, Sn, Al, Fe and Ni all have a significant impact on material strength.
Mn-containing alpha-beta brasses are known as high-strength brasses. They have great casting qualities but are also used for hot working.
Alpha-beta brasses have a zinc content of up to 45%. Anything above is beta brass but it finds a lot less use.
- Personal account manager
- Quality assurance
- Payment terms for companies
- On-time delivery by Fractory
Bronze
Although people usually know bronze as a uniform meaning, there are several classifications. Bronze alloys all have different qualities depending on the alloying elements used.
Tin Bronze

Applications: Springs, washers, coins, artsy bronze sheets, parts of pumps, pressure-resistant castings, bearings, etc.
The applications depend on the % of Sn used in the alloy. The maximum amount of Sn in alloys suitable for cold working is around 7%. Those copper alloys have good plasticity but are also easily work-hardened.
The maximum content of Sn is circa 20%. Starting from 5% of Sn, the structure of the alloy changes and they need some extra heat treatment. In turn, this causes a porous structure. That’s also the reason why they’re not suitable for other forming methods besides casting.
Adding phosphorus is necessary before the casting process. This helps with deoxidation. After the process, around 1% of phosphorus is left in the alloy, providing greater strength. Such alloys are called phosphor bronzes.
The main application for double phase tin bronzes is in making different types of bearings. Such a structure strikes a good balance where the α phase assures resistance to blows and knocks, while the hard and brittle chemical compounds bear the load and provide some wear resistance.
Zn and Pb are also sometimes present in tin bronzes. Zn improves the quality of castings, while also making the alloy cheaper. This kind of bronze is also known as gun-metal, as large guns were made of this material in the past.
Small amounts of lead help to improve a bronze’s mechanical properties for cutting. Large amounts (up to 25%) are present in lead bronzes that find use as bearing materials.
Aluminium Bronze
Applications: Coins, ship parts, marine hardware, sleeve bearings, pumps, valves, etc.
Aluminium bronzes have similar characteristics to tin bronzes. These alloys, mostly, have a single phase and are suitable for cold forming. This makes aluminium bronze a popular choice as a coin material. The content of aluminium is usually somewhere between 6…12%.
Double phase aluminium bronze finds use as a cast alloy or for hot working. Aluminium bronzes with an Al content of around 10% are used for making screw propellers, valves, pumps, etc. This metal is suitable for use in salty conditions or near the sea.
Cupronickels

Applications: Coins, marine equipment, electrical devices, heat exchangers, cooling systems, shipbuilding, etc.
Copper-nickel alloys are strong and plastic. Adding nickel (usually 2…30%) to copper makes the metal highly resistant to corrosion and gives it outstanding electrical conductivity properties.
Cu-Ni alloys have a nearly non-existent thermal expansion coefficient at 40…50% Ni. At the same time, electrical resistance is at a max. This kind of small thermal expansion coefficient is present until temperatures up to 500 °C.
That’s why constantan (Cu-Ni alloy with 45% of nickel) is used with electrical devices where large changes of temperature occur. For example, a typical use of constantan is forming a part of a thermocouple.
Corrosion resistant Cu-Ni alloys contain around 30% Ni along with a little bit of Fe and Mn which makes them especially stable in saltwater.



