Electroplating is a common surface finishing process in the manufacturing industry to coat a material (substrate) with another metal. In recent years, the process has undergone many advances, making it much more accurate and capable of working with a wider range of materials.
In this article, we will explore the modern electroplating process to understand what it is, how it works, its benefits and limitations.
What Is Electroplating?
Electroplating is a manufacturing process in which a thin layer of metal atoms is deposited to another material through electrolysis. The metal added is known as the deposition metal, and the underlying material or workpiece is known as the substrate material.
By adding a layer of the desired metal, we can improve several physical, mechanical and chemical properties of the substrate, such as its strength, heat conductivity, electrical conductivity, abrasion and corrosion resistance.
Improving these properties can allow us to combine different metals to achieve properties that perfectly suit different applications.
How Does the Electroplating Process Work?
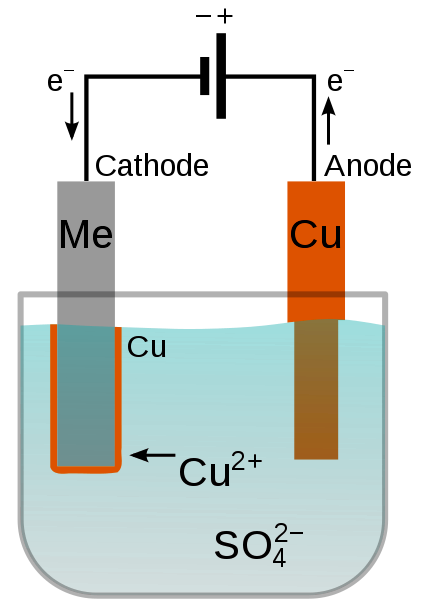
The electroplating process works on the principle of the electrolytic cell.
In this process, two metal rods are placed in an electrolyte. The rods act as electrodes when connected to the opposite terminals of a battery or power supply to create a potential difference. The electric current causes the electrolyte bath to disintegrate into dissolved metal ions, and the positively charged metal ions deposit on the negative electrode (cathode).
These positively charged ions are part of the electrolyte. As they get deposited on the cathode, their concentration in the electrolyte reduces. By choosing a suitable element for the anode, we can replenish the concentration of the positive ions.
For instance, if we need to coat brass with copper, the brass becomes the substrate. Connecting it to the negative terminal makes it the cathode. We use an electrolyte, such as a copper sulfate solution, that gives positive copper ions upon disintegrating. On the other end, we use a copper anode to replenish the electrolyte’s positive ions.
We can control the plate thickness, rate of metal deposition, surface finish, colour and many other factors by manipulating the process parameters. For example, using pure copper plates will give a better appearance than regular copper rods available in the market.
Using this process, the material can be coated with one or more metals.
Types of Electroplating Methods
Over the years, the electroplating process setup has evolved to suit different applications. By choosing a method in line with the application, the efficiency of the operation can be increased significantly.
To choose the right one, we must first understand the different types. Overall, electroplating methods can be divided into four major types. These are:
-
Mass plating
-
Rack plating
-
Continuous plating
-
In-line plating
Mass Plating
ARVE error: url: https://www.googleapis.com/youtube/v3/videos?part=snippet%2Cstatistics&id=7pC6BQZK-8Q&key=AIzaSyAQ7WFzTAUrOX-FjsIrFS3JwZBFzgIvloc Status code 200 expected but was 403.
As the name suggests, mass plating is used for mass-production applications. The method can handle a large volume of products that require thin coatings of metal.
A common type of mass plating method is known as barrel plating. In this method, the material to be coated (substrate) is dipped in a barrel containing the metal salt (electrolyte) and the anode of the coating metal.
The barrel plating setup is highly economical for small parts that need a uniform coating. As the barrel rotates, all the parts are cleaned, descaled and uniformly coated to a greater extent compared to rack plating.
This method is not recommended for parts that require a detailed finish without scratches and entanglement.
Mass plating is generally used for small but robust parts such as nuts, bolts and screws.
Rack plating
ARVE error: url: https://www.googleapis.com/youtube/v3/videos?part=snippet%2Cstatistics&id=h4D3oYHRTOA&key=AIzaSyAQ7WFzTAUrOX-FjsIrFS3JwZBFzgIvloc Status code 200 expected but was 403.
When the parts are larger than those suitable for mass plating, the rack plating method is used. In rack plating, the parts are mounted on racks and immersed in the chemical electroplating bath.
The rack plating process reduces the damage to delicate or fragile parts and coats the interior contours and deep crevices of parts, unlike mass plating.
This process is, however, more expensive than mass plating. But it makes up for it by providing a plated layer of much higher quality than a mass-plated product.
Rack plating is typically best for large, fragile and complex parts that require a plating of gold, silver, tin, copper or nickel.
Continuous plating
The continuous plating process is performed on exceptionally long parts, such as metal tubes, wires and strips.
In the case of thin strips, this process is also known as the reel-to-reel plating process. In this process, a long product is passed through the chemical bath at a specified rate. The end product’s quality is controlled by manipulating the process parameters and the time spent in the bath.
The reel of the product to be coated is uncoiled at the initial station, and once it passes the electrolyte/anode and gets coated, it is recoiled for easier storage and transport. Then further operations can be performed to stamp it into the required shapes.
In-line plating
The in-line plating method uses an assembly line for the metal plating operation. The metal passes through the various stations and automated machinery facilitates the chemical reaction.
Line plating is generally used for coating copper, zinc, chromium and cadmium. A variety of substrates can be coated with these metals through line plating. This method is relatively cheaper than other methods because a lower amount of chemicals is needed per piece.
- Personal account manager
- Quality assurance
- Payment terms for companies
- On-time delivery by Fractory
Suitable Materials
Electroplating is a versatile process owing to the fact that it requires only one property in the substrate: electrical conductivity.
Since this property is exclusively available with metals (barring a few exceptions), we could initially use electroplating only for metals. But with the advent of conductive sprays and coatings, it is now possible to coat non-conducting materials such as plastic and wood too.
As a result, today, there are many more materials that can be electroplated. The substrate material can greatly vary depending on the application.
Silver or gold plating is often used to improve the appearance. To improve properties such as bacterial resistance and conductivity, copper plating is a favourite. Copper electroplating also offers increased malleability, lubricity and corrosion resistance.
Similarly, when we need to improve corrosion and wear resistance simultaneously, we go for nickel plating. Nickel also improves the appearance of the product.
Some other metals that are normally used for coating in electroplating are chromium, cadmium, zinc, iron and titanium.
But the substrate and the coating must be chosen carefully. Not all materials combine with each other. For example, steel cannot be plated with silver right away. It must first be plated with copper or nickel before silver plating.
Benefits
The first modern electroplating plant was set up in Hamburg in the late 19th century. The intention was to improve the appearance. But as science understood the mechanism and benefits of electroplating, its applications for non-decorative purposes became common.
Today, we understand the true breadth of electroplating benefits. Let’s list them down for a better overall understanding.
Improved physical properties (colour, lustre, conductivity, low weight)
Electroplating improves physical properties such as colour, lustre and conductivity.
Colour and lustre provide cosmetic upgrades that are necessary for many day-to-day products as well as art applications.
Everyday appliances and kitchen products such as utensils, pans, cutlery, taps, kettles and other gadgets become much more attractive when coated with shinier metals such as copper, gold or silver. It also improves their functionality, as electroplated products are often easier to clean.
The appearance of artistic installations such as sculptures and figurines can also be improved by using electroplating. As a result, electroplating also finds use in art restoration and preservation projects besides new art creation.
Functionality can also receive a boost in technical applications involving electrical components such as antennas and integrated circuits. Although metals are already conductive, coating them with a better conductor improves the overall conductivity of the part while keeping costs low.
Costs are also reduced by the fact that non-metals can be used for electrical applications after electroplating. Besides having lower costs, non-metals also weigh less, which reduces the cost and difficulty related to the transport and storage of products.
Improved mechanical properties (tensile strength, bending strength, abrasion resistance, surface finish)
Electroplating also improves mechanical properties such as tensile strength, wear resistance and durability, depending on the application.
The small increase in tensile strength is enough to bridge the gap between the SLA resins of 3D printing (plastics) and metal alloys. The distinct strength characteristics allow the use of electroplated materials in applications where previously metals would have had to be used.
The metal skin on a plastic product, besides making the product lighter, also imparts excellent flexural strength characteristics.
We can also improve the surface finish using electroplating. This makes the products easier to handle and reduces friction.
All these improvements increase the short-term performance while also lengthening the lifespan of the products.
Improved Chemical Properties (Corrosion, Chemical, UV and Radiation Resistance)
The chemical properties of a material can also be enhanced by using electroplating. Properties such as corrosion resistance, resistance to chemicals and UV light are crucial in certain applications such as medical implants.
Typically, medical implants depend on precious metal coatings of gold, silver, platinum and copper for their corrosion protection, electrical conductivity, heat dissipation, non-toxic and antibacterial nature.
Chemical and corrosion-resistant products are also required for harsh service environments where the product is exposed to chemicals, moisture and seawater.
Limitations
Electroplating has certain disadvantages that prevent its use in some cases. Let’s evaluate these to get a complete picture.
Complex process
The process is far from simple and can be difficult to carry out reliably. A process would have to be set with predetermined parameters to obtain parts of a consistent quality. Mistakes in preparation and pretreatment can lead to defects, poor quality and capability of finished parts.
Electroplating cannot be used for all material combinations, as they may not combine well with the plating solution.
Long plating time
The plating time can be excessively long in some cases. The metal deposition rate can be increased by either increasing the power supply or the concentration of the electrolyte or both. But this will cause uneven deposition, which can be a dealbreaker in some cases.
The benefits are limited to the surface
By its nature, electroplating is only limited to the surface. Once the surface layer is scratched off, the product can lose some or all of the benefits provided by the process.
Hazardous nature
The process releases gases due to the reduction at the cathode. If these gases are of a hazardous nature, they pose considerable risks for personnel in the vicinity.
Hexavalent chromium exposure from chrome plating is an apt example of how hazardous the electroplating process can be.
Wrapping It Up
Electroplating is nothing short of an engineering wonder. In the past, we could only use it on metals, but that is no longer the case. Today, we can electroplate plastics, ceramics and even organic materials such as leaves and flowers.
However, it still remains a very difficult process to execute consistently. This is why engineers and designers should turn to electroplating service providers for their expertise. Fractory’s sales engineers have plenty of experience in planning and executing custom projects, so don’t hesitate to get in touch.
FAQ
How do I identify the positive and negative terminals of the power supply in the electroplating solution?
It is very important to maintain the right polarity during electroplating. If for some reason you are not able to identify the anode (positive electrode) from the cathode (negative electrode), remember that the bubbles are generated on the cathode during the reaction.
This tells us that the electrode with the bubble formation is connected to the negative terminal of the power supply.
What is the difference between electroplating and electropolishing?
Electropolishing is basically the reverse operation of plating. Instead of adding material, electrochemical polishing removes it. In the electropolishing process, the workpiece is the anode, contrary to electroplating, where the workpiece is the cathode. Thus, electropolishing is also known as the reverse plating process.
What is electroless plating?
Electroless plating works on the principle of an electrochemical cell. A chemical reaction causes the deposition of one material on another without the need for an electric current. The coating metal is usually a metal or a metal alloy and the substrate could be either a metal or non-metal such as plastic, ceramic, glass, etc.
What is electroforming?
The electroforming process refers to the use of electric current across a chemical bath to form solid models with intricate cavities. The process is similar to electroplating except that instead of a surface, we are building a solid article with a complex cavity.
It uses a template known as the mandrel. The mandrel is dipped in the electrolyte and the electrolytic reaction forms a layer of the deposition metal on the mandrel in the negative shape of the mandrel.





